R & D
R & DResearch & Development
Innovation Promise
In the dynamic landscape of the pharmaceutical industry, Research and Development (R&D) plays a pivotal role as the driving force behind innovation and progress. It serves as the crucible where groundbreaking ideas and scientific advancements converge to shape the future of healthcare.
R&D is the cornerstone of our quest for excellence, propelling us to explore new frontiers in drug development, enhance existing formulations, and bring novel solutions to the market. With a dedicated team of experts and cutting-edge technologies, our R&D initiatives not only elevate our products but also contribute to the collective advancement of the pharmaceutical field, ultimately benefiting patients worldwide.
In addition, our R&D department stands as a testament to our commitment to quality and innovation. Rooted in transparency, we proactively engage in the continuous development of products, adhering meticulously to international standards and guidelines. The overarching goal of our R&D efforts is the creation of both new and generic products that uphold the highest standards of quality. We channel our focus into the development of high-quality bioequivalent formulations and novel products, ensuring they not only meet but exceed expectations in terms of efficacy and safety.
Ensuring Compliance Excellence
We commit to regulatory compliance, ensuring our research and development efforts align with stringent industry standards and guidelines, fostering trust and reliability in our products.
Pioneering Ingenuity
Our commitment extends to pioneering breakthroughs and innovative solutions, driving us to explore uncharted territories in pharmaceutical research and development, constantly pushing the boundaries of what's possible.
Upholding Transparency and Integrity
In our pursuit of excellence, we uphold the highest standards of integrity and transparency, fostering a culture of openness and trust in every facet of our research and development processes.
Commitment to Product Excellence
Our commitment to delivering robust quality products is ingrained in our ethos, guaranteeing that each outcome of our research and development endeavors meets and exceeds the stringent benchmarks of excellence and reliability.
R&D Process Flow
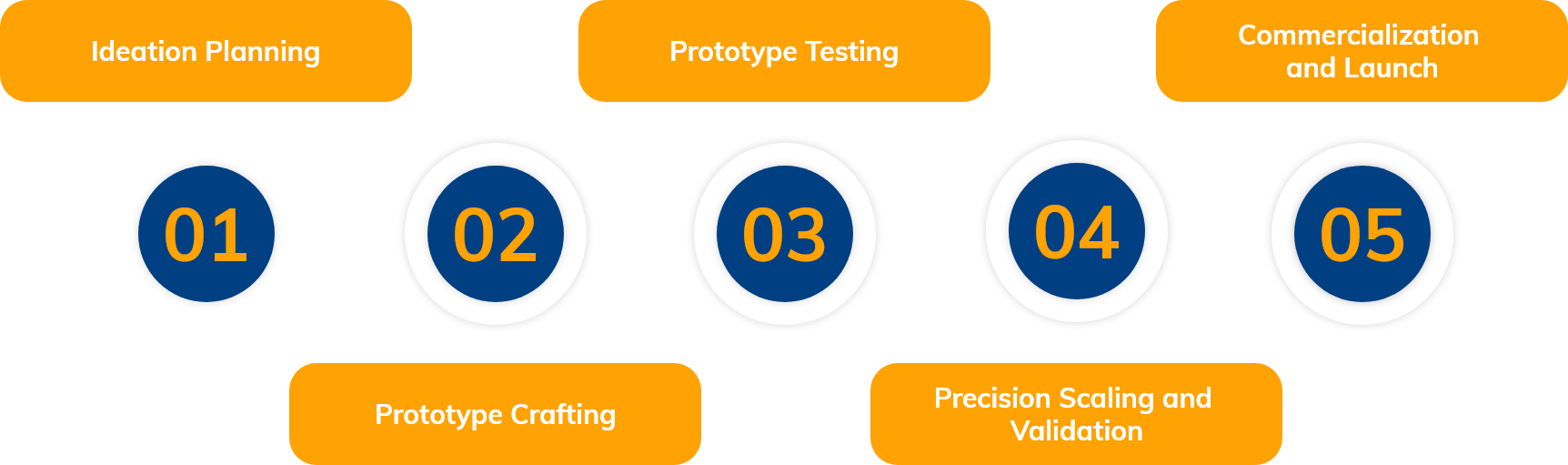
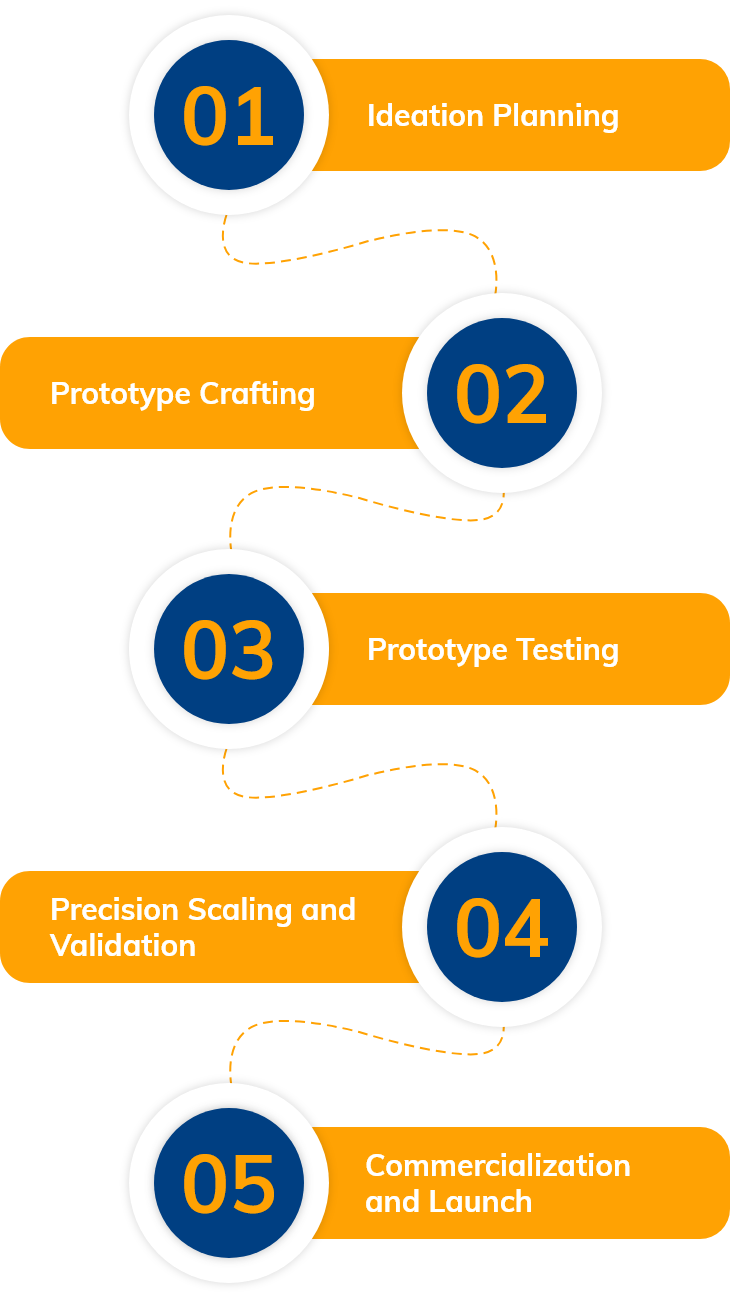
R&D Team

Our R&D team consists of knowledgeable and dedicated scientists that handle unique tasks such as:
Newer Formulation Development
Pioneering innovative drug formulations for cutting-edge healthcare solutions.
Analytical Development
Precision-driven analysis ensuring the highest quality and efficacy of formulations.
API Process Development
Expertise in refining and optimizing processes for Active Pharmaceutical Ingredients.
Method Validation
Rigorous validation to ensure accurate and reliable testing methodologies.
Packaging Development
Crafting packaging solutions that preserve and enhance the integrity of our products.
Regulatory Assistance
Navigating complex regulatory landscapes for seamless product approvals.
QA / QC
Upholding the highest standards of quality through rigorous assurance and control measures.
Project Management
Efficiently overseeing and executing projects with precision and strategic planning.
Stability Studies
Conducting thorough studies to ensure the stability and longevity of our formulations.
R&D Facility and Production
Mascot's cutting-edge R&D unit is a hub of innovation, boasting modern equipment and a dedicated team of scientists committed to pioneering new product development. Covering 500 square meters, our facility houses specialized sections for formulation development and analytical development, all maintained under an Air Handling Unit (AHU) system. This strategic setup includes distinct AHUs for various areas, ensuring meticulous control to prevent cross-contamination.
With a focus on precision, we maintain a low relative humidity (RH) and a controlled environment facility tailored for sensitive products. Our R&D features a segregated space for HPLC and other analytical instruments, integral to our work in developing new products approved by the Drug Controller General of India (DCGI) and bioequivalent formulations. We've expanded our product pipeline to address unmet market needs, conducting rigorous bioequivalence studies and clinical trials, ultimately securing DCGI approvals for manufacturing and marketing novel molecules and fixed-dose combinations.
